
How Long Can A Pokemon Stay In A Gym, How long can a Pokemon stay in a gym without berries, 1.26 MB, 00:55, 640, Ask About SPORTS, 2020-08-07T21:37:34.000000Z, 19, How long can a pokemon stay on a gym? | Pokemon GO Wiki - GamePress, pokemongo.gamepress.gg, 1440 x 1280, png, pokemon gym stay gamepress pokemongo, 20, how-long-can-a-pokemon-stay-in-a-gym, KAMPION
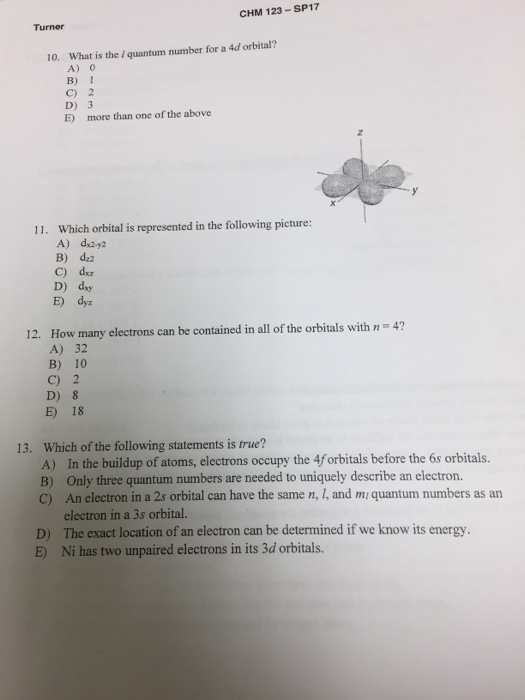
This means that the fourth energy shell can hold a maximum of 32 electrons. As you know, each orbital can hold a maximum of 2 electrons, as stated by pauli's exclusion principle. Secondly, how many electrons can fit in the orbital for which n 4 and l 2? N=4,l=2 means that is 4d orbital so it can accommodate maximum 10 electrons.
2 electrons l=1 => 4p subshell => max. 6 electrons l=2 => 4d subshell => max. 10 electrons l=3 => 4f subshell => max. So, theoretically n=4 means that 32 electrons can be. E) 32 formula =2n2 n=principal quantum no. View the full answer. How many electrons can be contained in all of the orbitals with n = 4? A) 2 b) 8 c) 10 d) 18 e) 32 6. Previous question next question.
Chapter 7 notes

Solved: How Many Electrons Can Be Contained In All Of The | Chegg.com

Quantum Numbers, Orbitals, and Probability Patterns | CK-12 Foundation
PPT - Electronic Structure of Atoms & Periodic Table PowerPoint

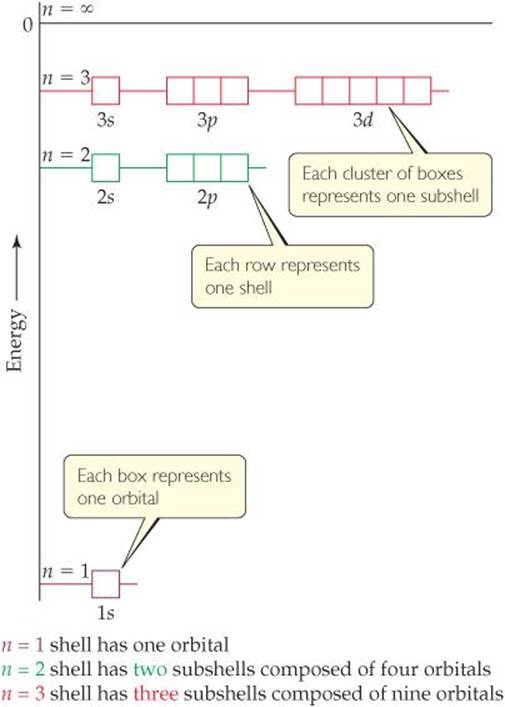
FIGURE 6.17 Energy levels in the hydrogen atom.
What is a Wave-Mechanical Model? | Study.com
Chemistry Archive | June 19, 2017 | Chegg.com
Solved: Consider The Quantum Number N=4 And L=2. Which Of | Chegg.com

Solved: What Is The L Quantum Number For A 4d Orbital. A) | Chegg.com
How many electrons with quantum numbers n=4 and l=1 can exist in an

EmoticonEmoticon