
How Long Can A Pokemon Stay In A Gym, How long can a Pokemon stay in a gym without berries, 1.26 MB, 00:55, 640, Ask About SPORTS, 2020-08-07T21:37:34.000000Z, 19, How long can a pokemon stay on a gym? | Pokemon GO Wiki - GamePress, pokemongo.gamepress.gg, 1440 x 1280, png, pokemon gym stay gamepress pokemongo, 20, how-long-can-a-pokemon-stay-in-a-gym, KAMPION
(a) 0. 2 m nacl (b) 0. 2 m cacl2 (c) 0. 2 m h2so4 (d) 0. 2 m nh3 (e) 0. 2 m al(no3)3 answer and explanation: Freezing point of a pure solvent depends on the amount of solute that gets dissolved in it. In order to determine which solution has the lowest freezing point, we need to look at the molality as well as whether the solute is ionic or covalent. Chemistry questions and answers.
0. 10 m cacl, b. 0,20 m naoh c. 0. 050 m k s04 od. 0. 010 m k, so. Question 8 which of these aqueous solutions would be expected to have the lowest freezing point assuming. So we have the same trend to than one, then three. So 213 is the order of increasing effective collective properties. However, when we're looking at freezing point where we want to put it in increasing, and so we know that the solution that's all you'd depress is the freezing point. So we need to reverse our order.
Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com

Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com

Assuming equal concentrations and complete | Chegg.com

Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com
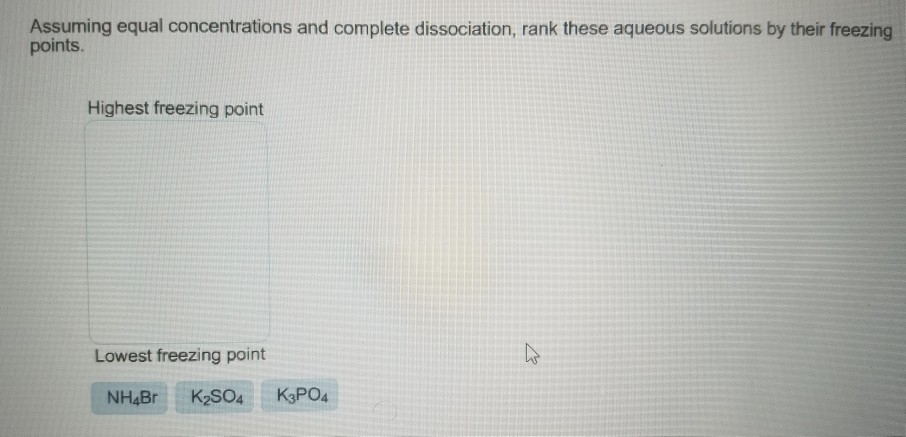
Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com
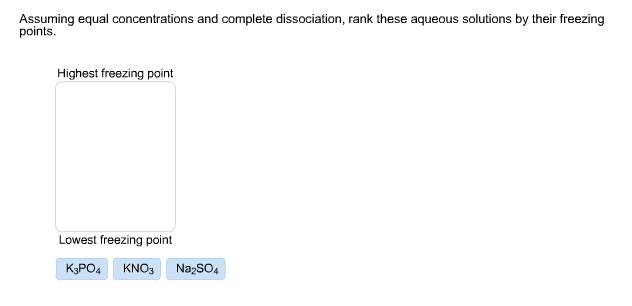
Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com
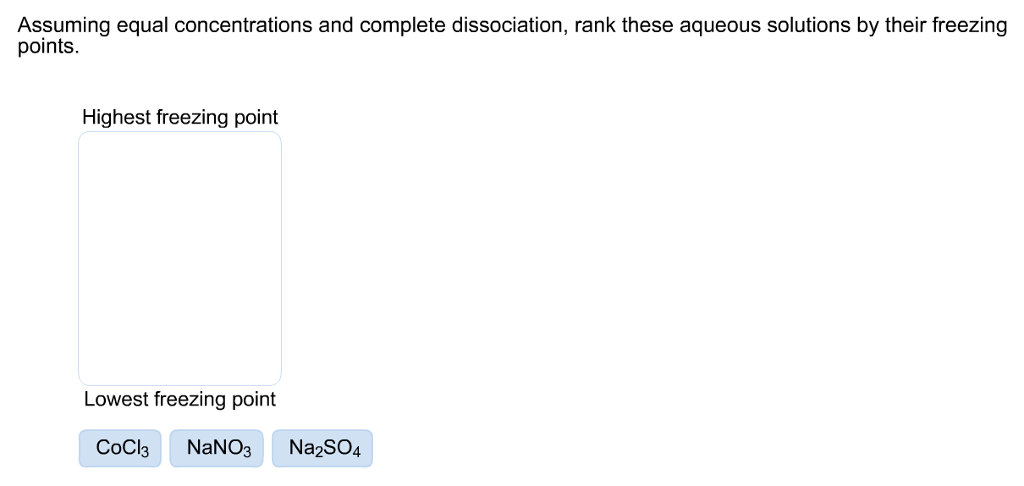
Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com
Precipitate forms No reaction Assuming equal | Chegg.com
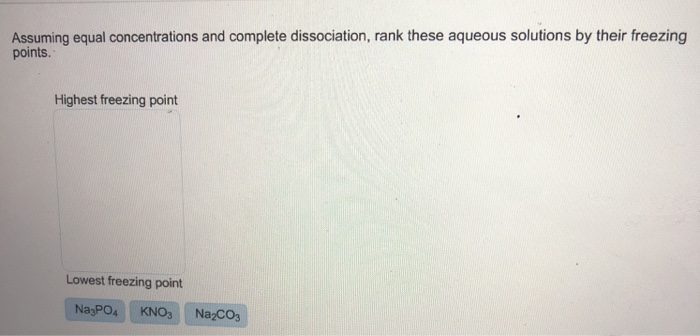
Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com
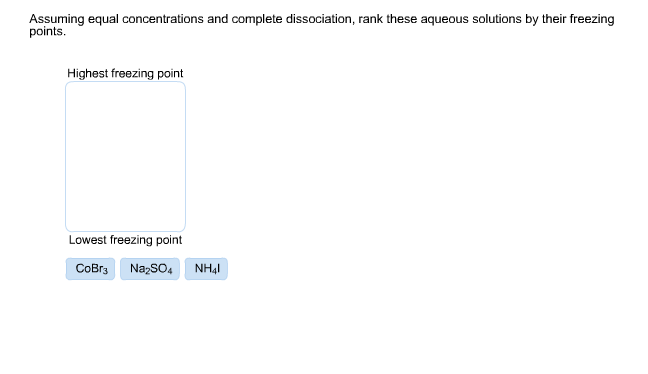
Solved: Assuming Equal Concentrations And Complete Dissoci... | Chegg.com
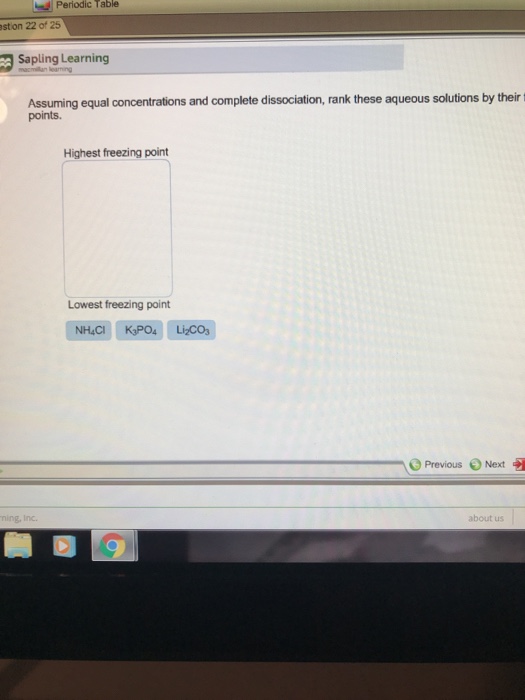
EmoticonEmoticon